Partner with Resyca to leverage our expertise, advanced platforms, and end-to-end services. Together, we can accelerate your product from concept to market and transform the future of drug delivery.
At Resyca, we help to bring advanced drug-device combination products to market faster and more efficiently.
With our expertise in inhalation product development, we deliver precise, patient-friendly solutions that support enhanced therapeutic outcomes. From early feasibility to full-scale commercial launch, we offer an end-to-end partnership designed around your unique development goals.
Every therapy is unique and that’s why our platforms are fully customisable.
Droplet size, plume velocity, dose, and deposition patterns can be precisely tuned to your formulation and clinical goals. By adapting our technologies to your product, we maximise therapeutic impact while ensuring patient safety, comfort, and compliance.
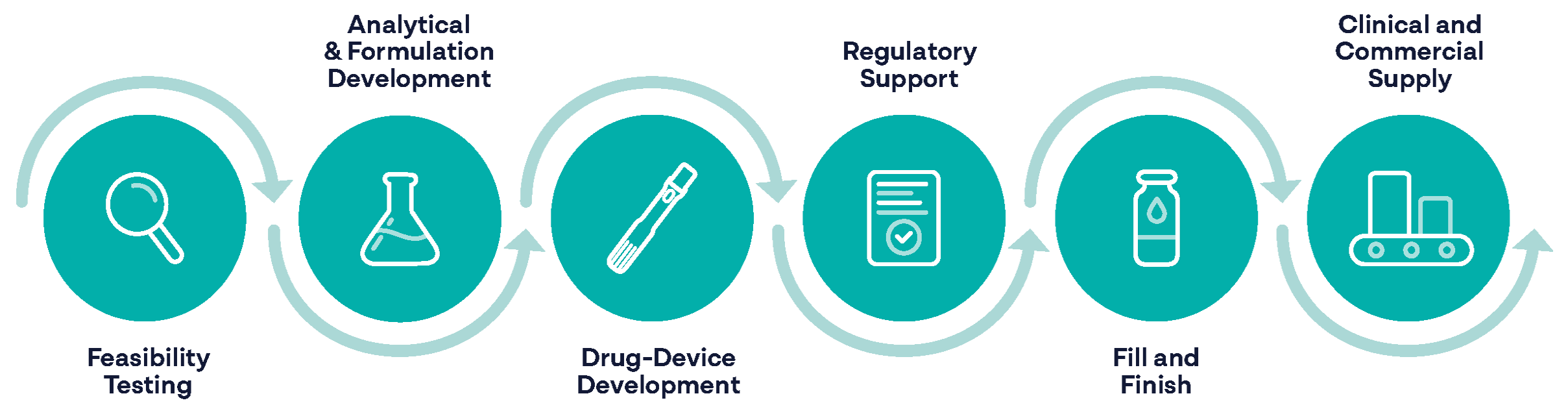
Resyca provides a comprehensive suite of services that guide your product from concept to market, including early-stage feasibility studies, formulation optimisation, and device selection.
Our regulatory experts help navigate compliance and submissions, ensuring a smooth path to approval. For clinical trials, we provide device supply, training, and performance monitoring. Commercial services, including fill-finish, labelling, packaging, and distribution, complete the integrated support needed for a seamless launch.
We are committed to accelerating your breakthrough from early development to commercialisation, tailoring our partnership to your specific needs.
With instant access to validated supply chains and quality-assured components, we help you navigate device strategy, regulatory pathways, and delivery science. By working as an integrated part of your team, we collaborate to shape the future of soft mist inhalation and nasal delivery concepts.
Let’s shape the future of soft mist inhalation together.