Recipharm, Medspray and Resyca enter into exclusive licence agreement to develop nasally-delivered drug products using proprietary soft mist technology
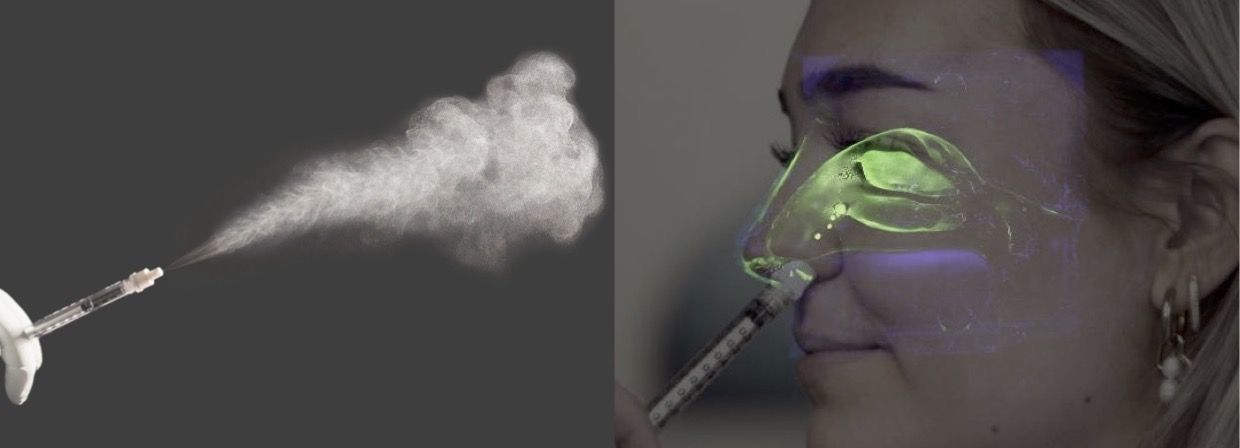
Partnership will help bring breakthroughs in the nasal delivery of fragile biologics and small molecules to market
Recipharm, a leading contract development and manufacturing organisation (CDMO) is pleased to announce an exclusive licence and collaboration agreement with Medspray and Resyca, to develop soft mist nasal delivery devices for single and combination drug products.
This collaboration extends the successful partnership between Recipharm and Medspray which resulted in the joint venture, Resyca; a company which is developing soft mist inhalers utilising Medspray’s nozzle technology to achieve high lung doses and less degradation of biological active
pharmaceutical ingredients.
Under the terms of the agreement, Resyca gains exclusive rights to Medspray’s proprietary spray nozzle technology for use in nasal devices and combination drug products. This collaboration enables Resyca to provide all aspects of end-to-end solutions for both oral and nasal inhalation, leveraging soft mist technology.
Han van Egmond, CEO at Medspray said: “Extending this agreement involves combining our cutting-edge nasal technology with Resyca’s product development capabilities. This collaboration
with Resyca will enable us to bring innovative solutions to the market that will redefine the landscape of nasal drug delivery.”
It is anticipated that the enhanced collaboration will result in breakthroughs in the nasal delivery of fragile biological formulations, such as mRNA vaccines for pandemic applications.
“We are thrilled to enter into this extended strategic partnership with Medspray,” said Remko Beimers, CEO at Resyca. “This move aligns perfectly with our mission to pioneer advancements in aerosol drug delivery systems. The exclusive licence from Medspray will significantly strengthen our position in the market, allowing us to bring innovative and effective soft mist nasal delivery devices to patients worldwide together with our pharma customers.”
Recipharm, as a key player in the pharmaceutical CDMO industry, recognises the potential impact of this collaboration on the advancement of nasal drug delivery. “We are proud to be associated with Medspray and Resyca in this landmark licensing agreement,” said Chris Hirst, President, Advanced Delivery Systems at Recipharm. “As a CDMO committed to driving innovation, we look forward to supporting both companies in bringing their pioneering soft mist nasal delivery devices to the market to improve patient experience and expand what is possible via the nasal delivery route.”
About Bespak®
Bespak is a global contract development and manufacturing organisation (CDMO) focused on inhaled and nasal drug delivery devices and drug-device combination products. Bespak develops and manufactures finished pharmaceutical products, as well as being a leading global supplier of drug delivery devices and componentry to the pharmaceutical industry. With a long history in the development and commercial supply of pressurised Metered Dose Inhalers (pMDIs), Bespak supplies a major proportion of the world’s pMDI dosing valves and actuators, and also specialises in the industrialisation and high-volume manufacture of complex dry powder inhaler (DPI) devices.
Headquartered in Holmes Chapel, UK, the company’s service offering spans early-stage feasibility, analytical services and product development, from pilot-scale, through to clinical supply and commercial-scale drug product fill-finish, device and component manufacturing.
More information www.bespak.com
About Medspray BV
Medspray is a privately owned company in Enschede, the Netherlands. With its 40 employees Medspray focuses on the development and manufacturing of micro spray nozzles which are able to deliver soft mists. Medspray’s slogan is: `tiny technology for a sustainable future’. Medspray aims to contribute to a sustainable world by developing innovative spray nozzles for user-friendly health and physical care products. By making propellants redundant and increasing the user-friendliness for various purposes, especially where a slow-moving soft spray is required. Thanks to the high-tech nozzle chips, sprays can be tailored to suit any application, from inhalable aerosol clouds, to gentle nasal sprays, to cosmetic or fragrance sprays that do not feel wet on your skin.
More information: www.medspray.com
About Resyca®
Resyca® was founded in 2020 and is a joint venture between Bespak and Medspray. Resyca® specialises in the development and manufacturing of compact, user-friendly soft mist inhalation and soft nasal spray devices, incorporating the proprietary Medspray micro-nozzle technology. These devices are tailored for pulmonary and nasal applications, utilising standard pre-filled syringes / primary packaging and filling lines. As the soft mist development centre within Bespak, Resyca offers comprehensive services spanning from early-stage development through to commercial production, inclusive of filling, labelling, and packaging.
For further information and interview opportunities with Resyca®, please contact:
Bernhard Müllinger at Resyca BV
Email: info@resyca.com
Website: www.resyca.com