Stevanato Group collaborates with Resyca ® to develop and manufacture pre-fillable syringes for use in a new soft mist inhaler for the inhalation of sensitive biological products
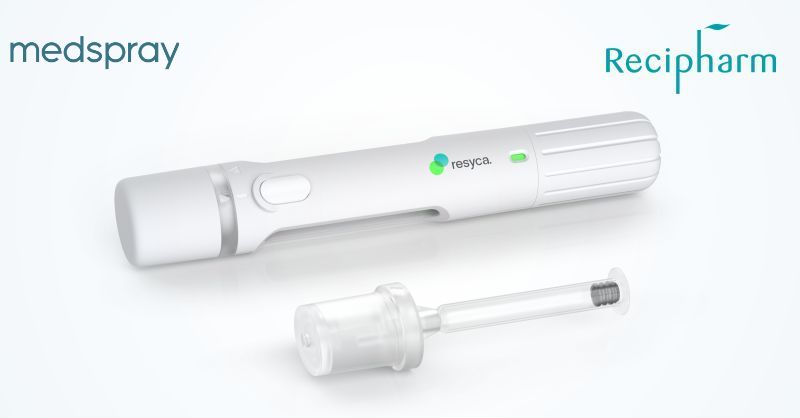
The new strategic collaboration aims to provide innovative primary packaging to pharmaceutical and biopharmaceutical companies using Resyca®’s proprietary soft mist inhalers.
Stevanato Group S.p.A. (NYSE: STVN) a leading global provider of drug containment, drug delivery, and diagnostic solutions to the pharmaceutical, biotechnology, and life sciences industries, today announced a collaboration with Resyca® BV (a joint venture between Recipharm and Medspray). Under the agreement, Stevanato Group will lend its 70+ years of manufacturing experience to support the development and production of pre-fillable syringes for use in Resyca®’s soft mist inhalers. As part of this collaboration, Stevanato Group will provide and manufacture its glass pre-fillable syringe Alba® assembled with the Integrated Spray Module (ISM™) of Resyca®’s soft mist inhaler technology, the Pre-Filled Syringe Inhaler (PFSI™).
This collaboration highlights Stevanato Group’s capabilities as a one-stop shop offering contract drug manufacturing organizations support in their drug development programs, from the clinical phase through to market release. Stevanato Group’s Alba® syringes feature an internal coating based on silicone oil, which is cross-linked with the glass surface, thereby minimizing sub-visible particle release and aiming to ensure superior performance during delivery.
The combination of the Alba® syringe and Resyca® ‘s innovative soft mist inhaler technology delivers sensitive drug products more efficiently to the respiratory airways and provides biopharma companies with a containment solution featuring enhanced stability and safety. With Stevanato Group taking responsibility for the complete primary drug package, Resyca®’s focus is on the design and manufacture of the spray technology, the inhalation device, and the fill and finish of the product.
Resyca®’s soft mist inhaler (PFSI™) is developed, manufactured, and licensed by Resyca® and is supplied as a pre-filled and ready-to-use inhalation device.
Bernhard Muellinger, General Manager and Chief Operational Officer at Resyca®, said: “By leveraging Stevanato Group’s integrated capabilities, from plastic injection molding and assembly capabilities to comprehensive scientific and analytical support services, we will be able to offer a turnkey solution, and accelerate and de-risk our pharmaceutical customers’ development programs. This is particularly true for novel inhaled biological products.”
“Biopharma companies are advancing patient care with new, innovative treatments, particularly in biologics and mRNA therapies. These products require specialized, high-performance drug containment systems, like our Alba® syringe platform, together with patient-centric drug delivery devices like the PFSI™. Thanks to our integrated end-to-end capabilities we are able to support our customers at scale with a comprehensive system solution,” said Mauro Stocchi, CBO of Stevanato Group.
Franco Moro, CEO of Stevanato Group, noted: “Creating a network of strategic collaboration partners is an important element of our long-term strategy to match customers’ needs and address self-administration trends in patient care with user-friendly drug delivery devices that provide variable and accurate dosing. This agreement marks another key step in broadening our high-value solutions and integrated capabilities as we continue to diversify and enhance our presence in the drug delivery market of pen injectors, auto-injectors, inhalers, and wearable pods”.
About Medspray
Medspray is a privately owned company in Enschede, the Netherlands. With its 40 employees Medspray focuses on the development and manufacturing of micro spray nozzles which are able to deliver soft mists. Medspray’s slogan is: ‘tiny technology for a sustainable future’. Medspray aims to contribute to a sustainable world by developing innovative spray nozzles for user-friendly health and physical care products. By making propellants redundant and increasing the user-friendliness for various purposes, especially where a slow-moving soft spray is required. Thanks to the high-tech nozzle chips, sprays can be tailored to suit any application, from inhalable aerosol clouds, to gentle nasal sprays, to cosmetic or fragrance sprays that do not feel wet on your skin.
More information: www.medspray.com
About Bespak®
Bespak is a global contract development and manufacturing organisation (CDMO) focused on inhaled and nasal drug delivery devices and drug-device combination products. Bespak develops and manufactures finished pharmaceutical products, as well as being a leading global supplier of drug delivery devices and componentry to the pharmaceutical industry. With a long history in the development and commercial supply of pressurised Metered Dose Inhalers (pMDIs), Bespak supplies a major proportion of the world’s pMDI dosing valves and actuators, and also specialises in the industrialisation and high-volume manufacture of complex dry powder inhaler (DPI) devices.
Headquartered in Holmes Chapel, UK, the company’s service offering spans early-stage feasibility, analytical services and product development, from pilot-scale, through to clinical supply and commercial-scale drug product fill-finish, device and component manufacturing.
More information: www.bespak.com
About Stevanato Group
Founded in 1949, Stevanato Group is a leading global provider of drug containment, drug delivery and diagnostic solutions to the pharmaceutical, biotechnology and life sciences industries. The Group delivers an integrated, end-to-end portfolio of products, processes and services that address customer needs across the entire drug life cycle at each of the development, clinical and commercial stages. Stevanato Group’s core capabilities in scientific research and development, its commitment to technical innovation and its engineering excellence are central to its ability to offer value added solutions to clients.
More information: www.stevanatogroup.com
About Resyca®
Resyca® was founded in 2020 and is a joint venture between Bespak and Medspray. Resyca® specialises in the development and manufacturing of compact, user-friendly soft mist inhalation and soft nasal spray devices, incorporating the proprietary Medspray micro-nozzle technology. These devices are tailored for pulmonary and nasal applications, utilising standard pre-filled syringes / primary packaging and filling lines. As the soft mist development centre within Bespak, Resyca offers comprehensive services spanning from early-stage development through to commercial production, inclusive of filling, labelling, and packaging.
For further information and interview opportunities with Resyca®, please contact:
Bernhard Müllinger at Resyca BV
Email: info@resyca.com
Website: www.resyca.com